Cellular Senescence and Pathophysiology
Our laboratory investigates cellular and molecular mechanisms that underlie human ageing and its associated pathologies. Our research is relevant to advancing the understanding and treatment of pathological processes, such as atherosclerosis and neurodegeneration, as well as the syndrome of frailty.
Originally known as the Cellular Senescence and Vascular Biology Group, the laboratory was first established in 1996 at University College London under the direction of
Professor Jorge D. Erusalimsky. In 2006, Jorge relocated his laboratory to Cardiff Metropolitan University, and in 2016 with the joining of
Dr Claire Kelly from Cardiff University and its expansion into cognate areas, the group was re-branded under its current name.
An important activity in the group is the training of graduate and postgraduate students, with the goal of providing an in-depth understanding of the molecular basis of normal physiology, pathological mechanisms and of novel methods for investigation and therapy.
Research / Innovation Areas
Cellular Senescence in Ageing and Vascular Disease
Cellular Senescence is a damage and stress-response which locks up mitotically competent cells into an essentially irreversible form of growth arrest. Originally described in cultured cells, cellular senescence is also now known to occur
in vivo, where it has been linked to the process of ageing and to the development of age-related diseases. In 2000, we described the presence of senescent vascular cells in the arterial wall and since then we have been investigating the mechanisms that control this phenomenon in endothelial cells1-,5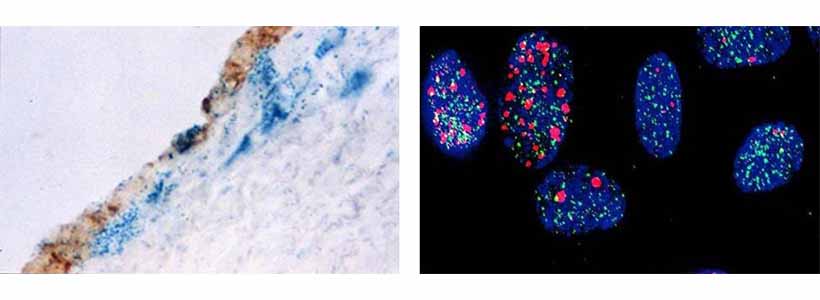
Image (left): Senescence-associated β-galactosidase staining (blue) in a section of a neointimal lesion which developed following denudation of the endothelium. The re-grown endothelial cells are stained brown. Note that some endothelial cells are senescent and the underlying blue cells are senescent vascular smooth muscle cells.
Image (right):
Endothelial cell nuclei showing increased DNA and telomere damage following depletion of SIRT6. DNA damage foci are shown in red, telomeres in green, and damaged telomeres in yellow.
1. Fenton M., Barker S., Kurz D.J. and Erusalimsky J.D. (2001) Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler. Thromb. Vasc. Biol. 21, 220-226
2. Erusalimsky J.D. (2009) Vascular endothelial senescence: From mechanisms to pathophysiology. J. Appl. Physiol. 106, 326-332
3. Cardus A., Uryga A., Walters G. and Erusalimsky J.D. (2013) SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction and senescence. Cardiovasc. Res. 97, 571-579
4. Villalobos L.A., Uryga A., Romacho T., Leivas A., Sánchez-Ferrer C.F., Erusalimsky J.D. and Peiró C. (2014) Visfatin/Nampt induces telomere damage and senescence in human endothelial cells Int. J. Cardiol. 175, 573-575.
5. Romero A., San Hipólito-Luengo A., Villalobos L.A., Vallejo S., Valencia I., Michalska P., Pajuelo-Lozano N., Sanchez-Perez I., León R., Bartha J.L., Sanz M.J., Erusalimsky J.D., Sánchez-Ferrer C.F., Romacho T. and Peiro C. (2019) The angiotensin-(1.7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell 18, e12913
Biomarkers of Frailty and Cognitive Decline
The increase in life expectancy of Western Societies is having major socio-economic and public health impacts. One crucial aspect emanating from this scenario is the increase in the number of frail people. Frailty is an age-associated syndrome characterised by a decline in several physiological systems and a decrease in resistance to stress. Frailty places elderly adults at increased risk of disability, falls, hospitalisation and premature death.
Currently, assessment of frailty relies primarily on measuring functional parameters such as cognitive function, exhaustion, weight loss, walking speed and grip strength. However, it is now increasingly recognised that the clinical utility of such parameters in terms of risk prediction, diagnosis and prognosis, is limited. Hence, there is an urgent need to ameliorate this situation. To this end, we are participating in a major European project, the FRAILOMIC initiative, which is measuring the levels of candidate biomarkers in the blood and urine of elderly individuals across the World1.
Working within this research programme we have demonstrated that the chemokine CCL11 (also known as Eotaxin-1) is associated with cognitive decline in rural dwellers2. We have also shown that the vascular inflammation-related protein sRAGE predicts mortality in frail older adults3. Ongoing work in this area aims to validate our findings in additional cohorts of older adults.
1. Erusalimsky J.D., Grillari J., Grune T., Jansen-Duerr P. Lippi G., Sinclair A.J., Tegner J., Vina J., Durrance-Bagale A., Minambres R., Viegas M. and Rodriguez-Mañas L. (2016) In search of ‘omics’-based biomarkers to predict the risk of frailty and its consequences in older individuals: The FRAILOMIC initiative Gerontology 62, 182-190.
2. Butcher L., Pérès K., André P., Morris R.H., Walter S., Dartigues J-F., Rodriguez-Mañas L., Feart C. and Erusalimsky J.D. (2018) Association between plasma CCL11 (eotaxin-1) and cognitive status in older adults: Differences between rural and urban dwellers. Exp. Gerontol. 113, 173-179
3. Butcher L., Carnicero J.A., Gomez Cabrero D., Dartigues J-F., Pérès K., Garcia-Garcia F.J., Rodriguez-Mañas L. and Erusalimsky J.D. (2019) Increased levels of soluble Receptor for Advanced Glycation End-products (RAGE) are associated with a higher risk of mortality in frail older adults. Age and Ageing. 48, 696-702
Regenerative Medicine for Neurodegenerative Diseases
Neurodegenerative diseases such as Parkinson's and Huntington's disease are characterised by focal cell loss in the central nervous system. Despite an increasing understanding of the pathophysiology behind these devastating diseases, as yet there is no known cure. 'Proof of principle' studies have shown that cell replacement therapy is a viable approach to treating these conditions. To overcome the limitations of this work, our current research is looking at adapting alternative cell sources as the donor tissue1,2. Dr Claire Kelly, the lead investigator in this area, is a board member of The Network for European CNS Transplantation and Restoration (NECTAR), an organisation that brings together European groups who share the common goal of protecting, repairing and restoring the central nervous system damaged through degenerative disease or injury. For more information on Nectar, click here .
.
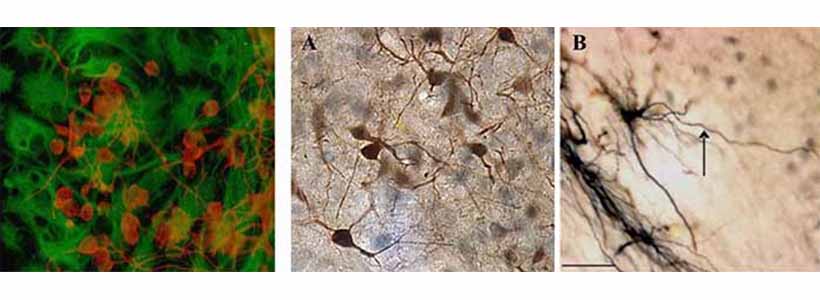
Left Image: Neural cells expanded in culture and stained for β-III tubulin (red) and glial fibrillary acidic protein (green).
MIddle and Right Images: Transplanted human foetal cells in an experimental model of Huntington's Disease differentiated into neurons (A) and more specifically into medium spiny neurons (B).
Of particular interest to our group is the role of inflammation in these diseases and how it contributes to their pathophysiology. Microglia cells are the resident immune cells of the brain that can acquire a pro- or anti-inflammatory character. There is a high abundance of these cells in the brain of diseased patients but their role and how they impact on the ability of transplanted cells to survive is unknown3.
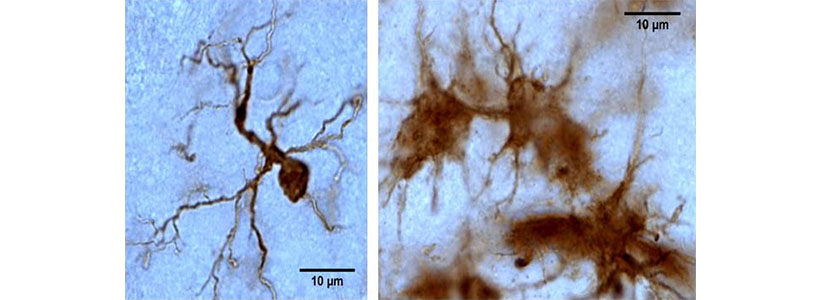
Microglia cells stained positive for Iba1. The cells acquire different morphologies with the classical appearance being the ramified, scavenging cell (left). In the activated state these cells appear clumped (right).
1. Kelly C.M. and Caldwell M.A. (2018) Derivation of Neural Stem Cells from the Developing and Adult Human Brain. Results Probl Cell Differ. 66, 3-20.
2. Noakes Z., Keefe F., Tamburini C., Kelly C.M., Cruz Santos M., Dunnett S.B., Errington A.C. and Li M. (2019) Human Pluripotent Stem Cell-Derived Striatal Interneurons: Differentiation and Maturation In Vitro and in the Rat Brain. Stem Cell Reports.12, 191-200.
3. Kodosaki E. (2019) The development of a novel model for microglia-like cells, and an investigation of the proinflammatory potential and response of CNS cells in vitro. Ph.D. Thesis. Cardiff Metropolitan University Research Repository (https://repository.cardiffmet.ac.uk/)
Immune cell infiltration into the brain and its role in neuro-inflammation
A main drive of our laboratory is to understand the role of the different cell types in neuro-inflammation. Within this remit, we are examining the contribution of immune cells that can cross the blood brain barrier. We have developed a cell model of these cells and characterised their differentiation in a neural environment in vitro. Ongoing work is looking at their functions in response to inflammatory stimuli and the effects on other neural cells.
Postgraduate Projects
Currently, the group has openings for self-funded MSc and PhD students in the areas of:
- Endothelial cell senescence (Professor Erusalimsky)
-
Neuroinflammation (Dr Kelly)
-
Stem cells and neurodegenerative disease (Dr Kelly)
-
Novel senolytic agents (Professor Erusalimsky)
Please email the relevant Principal Investigator for further details.
Group Members
 |  |
 |
Professor Jorge D. Erusalimsky, Professor of Biomedical Sciences
Group Lead | Senior Lecturer in Biomedical Sciences
Principal Investigator | Lecturer in Biomedical Science
Senior Investigator |
Collaborators
Internal
Professor Keith Morris, Department of Biomedical Sciences, Cardiff School of Sport & Health Sciences
Professor Phil James, Department of Biomedical Sciences, Cardiff School of Sport & Health Sciences
Dr Barry McDonnell, Department of Biomedical Sciences, Cardiff School of Sport & Health Sciences
External
Professor Concha Peiró, Universidad Autonoma de Madrid
Professor Carlos Sánchez Ferrer, Universidad Autonoma de Madrid
Professor Jorge Oksenberg, University of California San Francisco
Professor Leocadio Rodriguez Mañas, Hospital Universitario de Getafe, Madrid
Professor Catherine Feart, University of Bordeaux
Professor Anne Rosser, Cardiff University
Dr Jess Stevenson, Cardiff University
Professor Nick Allen, Cardiff University
Dr Eilis Dowd, NIUG Ireland
Professor Maeve Caldwell, Trinity College Dublin
Dr Andreas Heuer, Lund University
Professor Francisco Jose Garcia-Garcia, Hospital Virgen del Valle, Toledo
Dr Jose Antonio Carnicero, Fundacion para la investigación biomédica del Hospital Universitario de Getafe
Examples of Funding
European Commission FP7-Health-2012-Innovation-1 grant: "Utility of omic-based biomarkers in characterizing older individuals at risk for frailty, its progression to disability and general consequences to health and well-being - The FRAILOMIC Initiative." €11,940,343 in total; €441,613 to Cardiff Metropolitan University (2013-2018). Principal Investigator: Professor Jorge Erusalimsky.
Research Innovation Award: "The role of flavanoids in regulating the inflammatory response in a cellular model of Huntington's Disease." (2015-2019) Principal Investigator: Dr Claire Kelly.
Research and Enterprise Investment Fund: To define and characterise components in fenugreek seeds with beneficial properties in regulating and preventing neurodegenerative disease." (2017-2018) Principal Investigator: Dr Claire Kelly.(£40k)
Wellcome Trust Innovation Strategic Support Fund: "iPS cells from fetal neural tissue - do they retain their epigenetic memory?" (2013-2014) Principal Investigator: Dr Claire Kelly. (£15k)
Industry-funded research (Jellagen): "Characterisation of cell growth and differentiation on jellyfish-derived collagen and comparison to standard cell matrices." (2019-2020) Principal Investigator: Dr Claire Kelly. (£10K)
