Cardiovascular Metabolism and Inflammation
Research in the Cardiovascular Metabolism and Inflammation Group centres on factors that influence the normal function of healthy blood vessels. A priority of the group is exploring the effects of obesity and related co-morbidities on the functioning of the cardiovascular system. There is also a cross-cutting theme of investigating the molecular basis of atherosclerosis, a disease that involves a diverse group of cells including macrophages and endothelial cells, and plays a major part in the pathogenesis of diseases such as Stroke and Type 2 Diabetes. The group uses a range of in vitro models and clinical cohorts to analyse novel biomarkers in the circulation and at both a cellular and sub-cellular level.
Research / Innovation Areas
Nitric oxide metabolites
Nitric oxide (NO) is a gaseous free radical involved in several homeostatic mechanisms in the body. Our group is interested in the beneficial role of NO in endothelial function and in the maintenance of vascular tone.
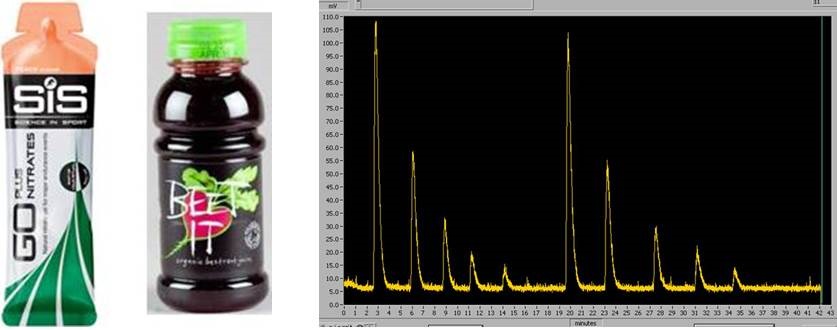
Can oral nitrate supplements enhance NO Bioavailability?
Table (right): Sodium nitrate standards measured using ozone-based chemiluminescence.
We use ozone-based chemiluminescence techniques to make high sensitivity measurements of NO metabolites such as nitrate, nitrite and nitrosothiols in blood and plasma. By profiling these metabolites in patients with different stages of cardiovascular disease, we can test whether oral nitrate supplementation enhances the circulating reservoir of NO metabolites, potentially improving the patient's cardiovascular function.
Extracellular vesicles
Extracellular vesicles (EVs) are submicron vesicles released from many cell types as intercellular communicators. EVs contain a bioactive cargo of lipids, proteins and nucleic acids that help to mediate their function and can also be used to identify EVs from complex biofluids.
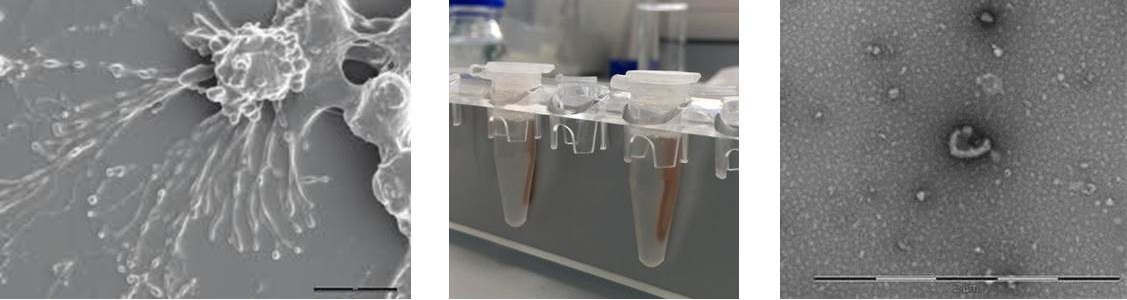
Image (left): Scanning electron microscopy of stimulated endothelial cells shedding EVs.
Image (centre): Magnetic bead separation of EVs from complex biofluids.
Image (right): Transmission electron microscopy of adipocyte-derived EV.
Our group is interested in the role of EVs in cardiovascular health and disease. We analyse markers carried by EVs circulating in plasma and those from primary adipocyte and endothelial cell cultures to test whether EVs can be used as disease biomarkers for cardiovascular disease and obesity. We also analyse the functional effects of these EVs on endothelial cells, leukocyte populations and isolated vessels. In addition to the biological character of EVs, we are also interested in aiding the standardisation of isolation and detection methods for EV research.
The role of inflammation in cardiovascular disease
Atherosclerosis plays a major part in the pathogenesis of diseases such as Type 2 Diabetes and its complications (cardiovascular diseases and neuroinflammatory diseases such as stroke). The pathogenesis of atherosclerosis involves a diverse group of cells including macrophages and endothelial cells. Our current interests are in how various lipids and glycosidic flavanoids can influence the inflammatory response through the regulation of macrophage polarization between the inflammatory M1 phenotype and the anti-inflammatory M2 phenotype. We are also investigating the interaction between endothelial cells and macrophages in angiogenic homeostasis. We measure these responses and interactions by monitoring the secretion of inflammatory mediators such as cytokines and the expression of regulatory transcription factors such as nuclear factor-κB (NF-κB) and the peroxisome proliferator activated receptors (PPARs), in particular PPARγ. We have recently begun to explore the modulation of EV release by saturated and unsaturated lipids and glycosidic flavanoids, and the effects of the resultant EVs on endothelial cells and macrophages.
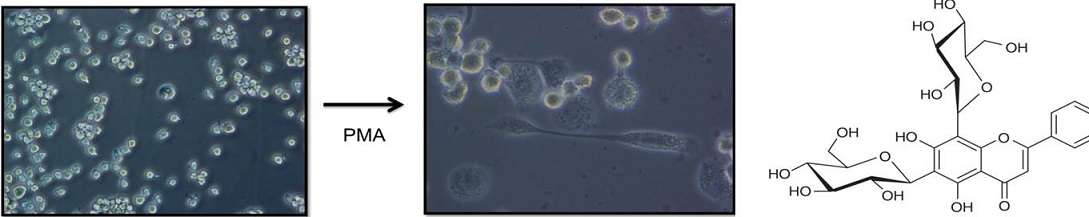
Images (left): Monocyte-derived macrophages: Differentiation of THP-1 cells using phorbol myristate acetate (PMA).
Image (right): Chemical structure of a di-glycosidic flavanoid.
The role of leukocytes in flow disturbances in the microcirculation and endothelial damage
Disturbances in the flow of blood cells and harmful interactions between blood cells and the blood vessel wall are important in the aetiology and pathophysiology of a range of diseases including cardiovascular disease and diabetes. Our research primarily focuses on how changes in the biochemistry and cytoskeletal function of cells may influence the cells biophysical properties and the flow of blood. We are able to measure the deformability of blood cells by measuring the flow through artificial models of the microcirculation using capillary-sized pores. We have also developed a model to analyse the change in leukocyte flow following alterations to the actin cytoskeleton in diseases such as diabetes. We are currently using these models to explore the role of physical activity and pharmacological intervention in normalising leukocyte biochemistry and biophysics.
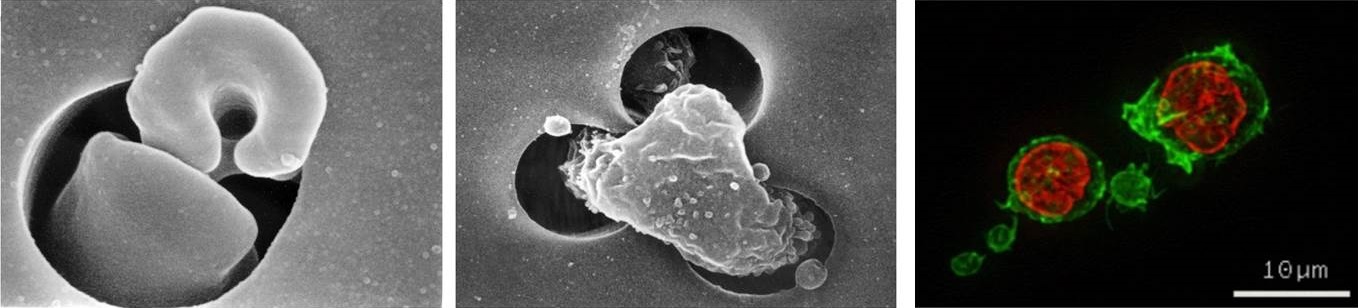
Image (left & centre): Scanning electron micrographs of a human erythrocyte (left) and a human leukocyte (centre) flowing through a capillary-sized pore.
Image (right): Fluorescence microscopy of f-actin polymerisation in human leukocytes. F-actin stained with FITC-phalloidin (green) and nuclear staining with propidium iodide (red).
Cardiovascular risk associated with exposure to particulate air pollution and engineered nanoparticles
Exposure to particulate air pollution and novel engineered nanoparticles presents significant risks to health. Air pollution is the world's largest environmental health risk, with
WHO estimating that 7 million people die annually as a result of exposure. The use of novel engineered nanoparticles is increasing, though their potential risks to health and the environment are unclear. Our research investigates the mechanisms by which exposure to air pollution increases cardiovascular risk, for example, by examining the effect of exposure to air pollution particles on leukocyte biochemistry and cytoskeletal function. We have recently begun experiments to monitor the interactions between leukocytes and a range of particulate pollutants, such as diesel exhaust particles and novel engineered particles including silver and iron nanoparticles.
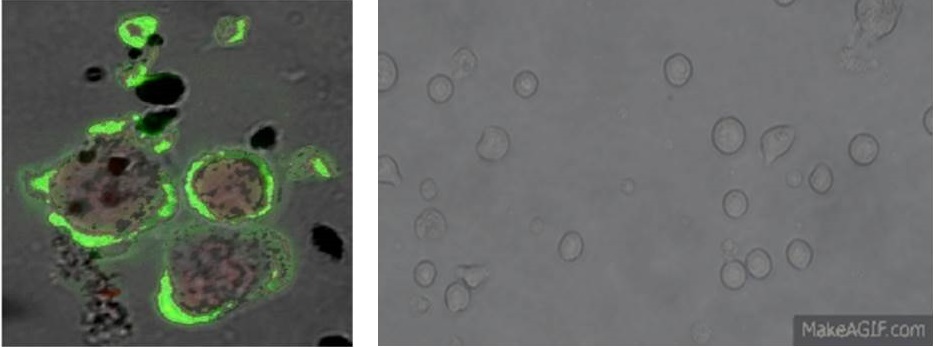
Image (left): Confocal microscopy of interaction between diesel exhaust particles and human leukocytes, f-actin stained with FITC-phalloidin (green).
Image (right): Time-lapse photography of increased cell motility of cultured THP-1 monocytes following exposure to engineered nanoparticles.
Group members
 |  |  |
Professor of Cardiovascular Metabolism/Associate Dean Research(Health) Group Lead | Professor of Biomedical Sciences & Biostatistics | Dr Maninder Ahluwalia
Senior Lecturer in Clinical/Human Genetics |
 |  | 
|
Senior Lecturer
Professor of Environment and Human Health, Faculty of Health, University of Plymouth and CEO & Founder, Molendotech Ltd
| Dr Shelley-Ann Evans
Senior Lecturer
Visiting Prof John Geen
Consultant Clinical Scientist, Head of Department, Clinical Biochemistry and Point of Care testing Services & Assistant Director for R&D at Cwm Taf Morgannwg University Health Board
| Dr Jessica Williams
Post-doctoral Research Associate
Professor of Endocrinology, Cardiff University
|
Students
Owen Griffith - PhD
Eleftheria Kodosaki - PhD
Mike Melhuish - PhD
Jamie Nash - PhD
Cory Richards - PhD
Mike Theodoulides - PhD
Cass Whelan - PhD
Collaborators
Dr Aled Rees, Division of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University
Dr Aled Clayton, Division of Cancer and Genetics, School of Medicine, Cardiff University
Dr Richard Anderson, Department of Cardiology, University Hospital of Wales
Dr Dev Datta, Lipid Unit, University Hospital Llandough
Professor Adrian Evans, Swansea University Medical School
Dr Kelly Bérubé, School of Biosciences, Cardiff University
Dr Timothy Higgins, School of Biosciences, Cardiff University
Professor Shareen Doak, Swansea University Medical School
Dr Martin Clift, Swansea University Medical School
Examples of Funding
Cardiff Metropolitan PhD Scholarship (Lead Supervisor). Mr Cass Whelan. The Role of Extracellular Vesicles Derived from Hypoxic Endothelial Cells in Patients with Cardiovascular Disease (2018-2021); £78,000.
Waterloo Foundation (Co-Applicant. Rees DA (P.I.)). Effect of high intensity interval training versus moderate-intensity steady-state training on mental health, cognitive and cardiometabolic outcomes in young women with Polycystic Ovary Syndrome: a pilot, randomised, controlled trial (2019-2021); £56,709.
KESS2 PhD Studentship. (Lead Supervisor). Mr Jamie Nash. Addressing the Platelet storage lesion - new approaches to optimising storage and function of donated platelets (2018-2021); £75,000
KESS PhD Studentship. (Co-Supervisor). Mr Owen Griffith. Development of an orally administered ovine polyclonal antibody based therapy (2017-2020); £75,000
HCRW/Stroke Implementation Group. Stroke Hub Wales, (2017-2021); £225,143.
Life Sciences National Research Network (Wales), Swansea University, Cardiff Metropolitan University PhD Studentship, £75,974 (2015-2018). "Mechanistic evaluation of the impact of superparamagnetic iron oxide nanoparticles conjugated with drugs (SPIONd) on intracellular signalling/homeostatic mechanisms. Principal Investigator: Professor Shareen Doak. Co-Investigators: Dr Rachel Adams, Dr Richard Webb.
Royal College of Physicians Clinical Research Fellowship to Dr Justyna Witczak, £120,417 (2015-2017). "Cell-derived microparticles as mediators of obesity-induced vascular disease" Principal Investigator: Dr Aled Rees. Co-Investigators: Professor Philip James, Dr Justyna Witczak.
British Heart Foundation Project Grant, £226,891 (2015-2018). "Extracellular vesicle transport in the circulation - a missing link between adipocytes and accelerated vascular dysfunction" Principal Investigator: Professor Philip James. Co-Investigators: Dr Aled Rees, Professor Marian Ludgate, Dr Aled Clayton, Dr Tim Hughes, Professor Valerie O'Donnell.
Cardiff University College of Biomedical & Life Sciences PhD Studentship, £75,000 (2014-2017). "Measurement of pathologically relevant microvesicle populations in obesity-driven cardiovascular disease" Principal Investigator: Professor Philip James. Co-Investigator: Dr Aled Rees.
National Institute for Social Care and Health Research PhD Studentship, £66,926 (2014-2017). "Can we prevent generation of pro-thrombotic and pro-coagulant extracellular vesicle populations in blood via a simple dietary intervention?" Principal Investigator: Dr Aled Rees. Co-Investigator: Professor Philip James.
